- Batteries, particularly lead-acid and mercury batteries, are among the most toxic materials on Earth due to their harmful chemical contents, such as lead, mercury, cadmium, and electrolytes. These substances pose severe environmental and health risks. For example, cadmium and nickel are known to be carcinogenic, while mercury in batteries releases toxic compounds that can easily spread through air, water, and soil, causing long-term ecological damage. In the case of lead-acid batteries commonly used in vehicles, lead is a highly toxic element that can contaminate soil and water if disposed of improperly.
- However, lead-acid batteries also serve as a model for successful recycling and the circular economy. Circular economy practices focus on minimizing waste and making the most of existing resources through reuse, recycling, and recovery. Lead-acid batteries are a prime example of this model. According to studies, lead-acid batteries boast a remarkable 99% recycling rate, which makes them one of the most successfully recycled consumer products globally (Newswire, 2017).
- The recycling process for lead-acid batteries involves several steps. First, when these batteries reach the end of their useful life, they are collected through local or state waste management programs. The batteries are then broken down into their constituent materials: acid, plastic, and lead alloys. The acid is neutralized, while the plastic components are recycled into new products. The lead alloys, which are the most valuable and hazardous part of the battery, are separated and purified. This secondary lead is then used to manufacture new batteries, completing the cycle of reuse.
- By recovering lead from used batteries and transforming it into new products, the recycling process significantly reduces the need for raw materials, conserves natural resources, and minimizes environmental impact. This closed-loop system not only helps reduce the environmental footprint of lead mining but also contributes to reducing lead contamination from improper disposal. Thus, lead-acid batteries, despite their toxicity, are an effective example of how recycling and circular economy principles can be applied to hazardous materials, ensuring sustainability and reducing waste. The following diagram illustrates the situation:

Fig. 1: Circular economy of lead-acid batteries
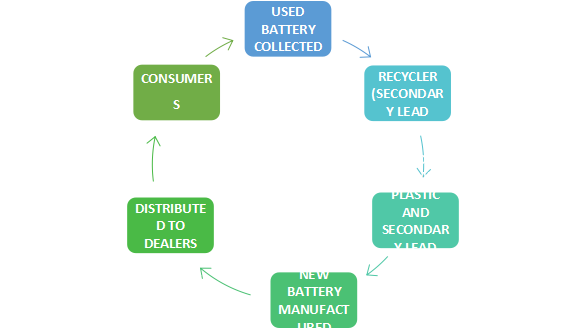
Fig. 2: Life cycle of lead acid batteries
- Lead-acid batteries are widely used in a variety of applications, including in households, industries, vehicles, boats, hospitals, generators, appliances, and even warfare equipment. Despite their usefulness, these batteries pose significant environmental and health risks due to their high lead content, a substance that is highly toxic. If not disposed of properly, lead can contaminate soil and water sources, leading to serious environmental and public health issues. The leakage of these batteries can also result in fires, explosions, poisoning, and further ecological damage. These dangers highlight the importance of proper disposal and recycling systems for such hazardous materials.
- The manufacturing process of lead-acid batteries begins with the extraction and processing of raw materials, including lead ore. Primary lead is extracted, then processed into lead plates and pastes, which are essential components in the construction of lead-acid batteries. These batteries are then transported to consumers for use. Once a lead-acid battery has reached the end of its useful life, it enters a recycling process where the materials—particularly the lead—are recovered and reused. In some cases, older batteries are refurbished for further use, while the lead content is recycled to create new batteries, reducing the need for primary lead extraction and limiting environmental damage.
- While lead-acid batteries are crucial for many industries and applications, their production and use contribute to a range of environmental issues. According to Zhang (2013), the life cycle environmental impact of lead-acid batteries includes global warming potential, acidification of ecosystems, respiratory problems, and negative effects on children’s health due to lead exposure. The most significant environmental toxicity arises during the extraction of raw materials, as lead is a highly toxic substance, and the processes involved in mining and refining lead are resource-intensive and damaging to ecosystems. Furthermore, improper disposal of these batteries at the end of their lives can lead to further contamination.
- In comparison to lead-acid batteries, mercury batteries have an even more hazardous life cycle in terms of manufacturing, use, disposal, and recycling. The collection and recycling of mercury batteries are fraught with complications due to the extreme toxicity of mercury, which makes them even more dangerous than lead-acid batteries.
- One way to mitigate the environmental impact of lead-acid batteries is by improving the mining and processing technologies used to extract lead. Eco-friendly mining techniques and more advanced mining equipment could reduce the environmental damage caused during the extraction phase. Additionally, using regenerated lead—which is lead that has been recycled from used batteries—can significantly reduce the environmental burden associated with these batteries. According to Chen, Lian, Li, and Kim (2017), the use of regenerated lead can reduce the overall environmental impact of lead-acid batteries by up to 69.5%. This reduction comes from the fact that regenerated lead requires much less energy to produce than primary lead, reduces the need for new mining, and minimizes the potential for environmental contamination.
- Incorporating regenerated lead into the production cycle not only helps make lead-acid batteries more sustainable but also enhances their environmental performance across their entire life cycle. By promoting better recycling systems and more sustainable mining practices, the environmental and health risks associated with lead-acid batteries can be greatly reduced, leading to a more sustainable approach to their widespread use.
References
Chen, S., Lian, Z., Li, S., & Kim, J. (2017). The Environmental Burdens of Lead-Acid Batteries in China: Insights from an Integrated Material Flow Analysis and Life Cycle Assessment of Lead. MDPI, 1-15.
Ecomena. (2016, Jul 30). The Problem of Used Lead-Acid Batteries. Retrieved from Ecomena: https://www.ecomena.org/managing-lead-acid-batteries/
Environmental protection. (2016, Feb 9). Ways to Reduce Our Overconsumption of Modern Technology. Retrieved from Environmental Protection: https://eponline.com/Blogs/Environmental-Protection-Blog/2016/02/Ways-To-Reduce-Our-Overconsumption-of-Modern-Technology.aspx
Newswire. (2017, Nov 15). Lead Battery Recycling Tops All Consumer Recycling And Provides A True Example Of A Circular Economy. Retrieved from Newswire: https://www.prnewswire.com/news-releases/lead-battery-recycling-tops-all-consumer-recycling-and-provides-a-true-example-of-a-circular-economy-300556631.html
Zhang, H. (2013). Life cycle assessment of lead-acid batteries. Chin. J. Environ. Manag, 39-48.


Your article helped me a lot, is there any more related content? Thanks!
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thank you everyone for your love and kind support